This is the 4th and final entry in a series about biological energy. In case you need a refresher, check the earlier entries linked below.
All caught up? Good, let’s go…
In part 1, we discussed what a calorie is – a measurement of energy. Raise your hand if you have ever studied the number of calories on a food nutrition label. This is essentially a measure of the energy content in food. More calories = more energy. We know that energy comes in the form of ATP which is generated by three main pathways in our bodies. Now it’s time to talk energy content in the food we eat.
There are three classes of nutrients that are large enough to provide energy. These three are called macronutrients or macros if you’re in the biz, and they are carbohydrates, fats, and protein. Because they are generally easy and fast to digest, carbohydrates are the body’s preferred fuel source. Chains of carbohydrates, such as starch and glycogen, are a long-term storage forms and provide reserves when needed. Protein is used to build the body’s structure (tissues and organs, for example) and assist with chemical reactions (as enzymes). Lastly, fats may be used for hormone production and also provide barriers around the cells in our bodies. Bonus points, fat can also be used as an energy source during the oxidative energy pathway.
If you take a set amount of each macro, such as 1 gram (g), you know there is a set number of calories present. For example, 1g of carbohydrates contains 4 calories. Same thing for protein, 4 calories. Fats on the other hand are more energy-dense and contain 9 calories per gram. Finally, it’s a safe assumption that everybody’s favorite adult beverage, alcohol, contains 7 calories per gram.
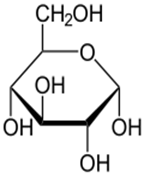
Glucose is the body’s preferred fuel
So now we know that food contains energy that is measured in calories and in order for our bodies to access that energy, we have to metabolize (break down) food. The carbohydrate glucose is our body’s favorite fuel so let’s take a closer look at how it is used. In its simplest form, glucose occurs as a single molecule – a structure with carbon atoms making up its 6-sided backbone.
Glycogen is a storage form of glucose
However, multiple glucose molecules can joint together to form more complex structures such as glycogen. The brackets around the middle glucose indicate glucose units 7-11 but could be any range of numbers depending on how big the chain is. Notice the branched structure of glycogen. This characteristic contributes to the bulk of muscle tissue.
When the body calls on this long-term storage to help meet energy needs, individual glucose units are clipped off the main chain and the process of glycolysis can begin. Below is a diagram showing the whole glycolysis pathway.
The overall reaction of glycolysis is:
Glycolysis is the breakdown of simple carbohydrates
Glucose + 2 NAD+ + 2ADP + 2Pi → 2 pyruvate + 2NADH + 2ATP + 2H2O + 2H+
Obviously this pathway is technical and dense but I wanted to show how glucose storage and subsequent breakdown contributes to energy production. Notice that there are several ATP molecules being shown in the reaction diagram and there are 2ATP on the right side of the overall equation. Glycolysis effectively generates 2 ATP. If you think this sounds like not much, you would be correct. This is the reason why glycolysis is effective in exercise lasting for relatively short periods.